Sinonasal Malignant Triton Tumor: A Case Report with Historical and Surgical Review
Senger JL, Kanthan SC and Kanthan R
DOI10.21767/2572-2107.100012
Senger JL1, Kanthan SC2 and Kanthan R3
1Department of Surgery, University of Alberta, 8440-112th Street, Edmonton, AB T6G2B7, Canada
2Department of Surgery, University of Saskatchewan, 103 Hospital Drive, Saskatoon, SK S7N 0W8, Canada
3Department of Pathology and Laboratory Medicine, University of Saskatchewan, 103 Hospital Drive, Saskatoon, SK S7N 0W8, Canada
- Corresponding Author:
- Senger JL
Department of Surgery, University of Alberta
8440-112th Street, Edmonton, AB T6G2B7, Canada.
Tel: 7809067221
Fax: 7804073409
E-mail: jennalynn@ualberta.ca
Received date: July 30, 2016; Accepted date: August 08, 2016; Published date: August 15, 2016
Citation: Senger JL, Kanthan SC, Kanthan R. Sinonasal Malignant Triton Tumor: A Case Report with Historical and Surgical Review. Head Neck Cancer Res. 2016, 1:2.
Abstract
Diagnosis of malignant peripheral nerve sheath tumors (MPNST) remains a challenge due to its rarity, morphologic similarities with other sarcomas, and paucity of specific immunohistochemical markers. MPNST associated with skeletal muscle differentiation are termed “malignant triton tumor” (MTT). This manuscript reviews sinonasal malignant triton lesions. A 26 year-old female presenting with unilateral nasal obstruction was found on imaging to have opacification of the left nasal passage and sinuses due to assumed nasal polyps. A nasal polypectomy was performed. Pathological examination confirmed a nerve sheath tumor with skeletal differentiation in keeping with MTT. A completion radical resection was undertaken. A comprehensive meta-analysis of all MTT cases in the published English literature was undertaken with a focused review on sinonasal MTTs. MTT is uncommon in the nasal sinuses. Pathological evaluation with immunohistochemistry is the diagnostic tool for differentiating malignant Triton tumors from routine MPNSTs. Early and accurate diagnosis is critical in best patient management as complete tumor resection with negative surgical margins improves patient survival.
Keywords
Malignant triton tumor; Sinonasal tract; Rhabdomyoblastic differentiation; Malignant peripheral nerve sheath tumor
Introduction
Malignant peripheral nerve sheath tumor (MPNST) is a rare form of soft tissue sarcoma that is most commonly associated with neurofibromatosis. MPNST account for 5-10% of all soft tissue sarcomas [1,2], and arise from either pluripotent cells of neural crest origin or Schwann cells [3]. These can differentiate along various mesenchymal cell lines including epithelial, which is usually histologically benign; the commonest mesenchymal differentiation however is sarcomatous [2]. This subset of MPNST composed of rhabdomyosarcomatous mesenchymal differentiation on a background of Schwann cells is termed malignant triton tumor (MTT).
Triton tumors are rare, representing only 5% of all MPNSTs [1]. The majority of MTTs (60-70%) are proposed to arise in conjunction with neurofibromatosis type-1, and the remainder are sporadic lesions; the former being most common in young males and the latter in older females [1]. MTTs are uncommon in children [4]. MTTs commonly occur in large nerves or plexi; rare sites include the esophagus, lung, thyroid, bladder, kidney, rectum and sinonasal tract. These demographics are based on isolated case reports/case series.
This review includes a case report and a comprehensive metaanalysis of all reported cases of sinonasal MTT in the published English literature.
Case Report
A 26 year old female presented with large nasal polyps obstructing the left nasal cavity with extension into the nasopharynx. Past medical history was unremarkable. Family history was negative for neurofibromatosis-1 with no stigmata on physical exam. CT scan showed opacification of the left nasal sinuses including maxillary, ethmoidal, and frontal.
Exploratory endoscopic curettage was performed with septoplasty and removal of multiple left-sided ‘nasal polyps’. Histopathological examination identified a cellular spindled neoplasm arranged in sheets and fascicles with focal myxoid change. A scattered area of rhabdomyoblastic differentiation was observed that was confirmed by immunohistochemistry: desmin positivity in the rhabdomyoblastic area, S100 positivity in the spindle cells, with a low Ki67 proliferative index. The final diagnosis was a triton tumour. Radical resection including anterior and posterior ethmoidectomies, middle meatal antrostomies, and middle turbinectomies were undertaken. Pathological examination of the final resected specimen confirmed negative surgical margins.
Surveillance biopsies conducted at yearly intervals remained negative for residual tumor. Follow-up with yearly CT scans do not show any evidence of recurrent disease at 6 years.
Literature Review
A comprehensive review of the published literature using the search terms “triton tumor” and “malignant peripheral nerve sheath tumor AND rhabdomyosarcoma” were used to identify references from which secondary reports were extrapolated. Articles in any language other than English are not included in this review.
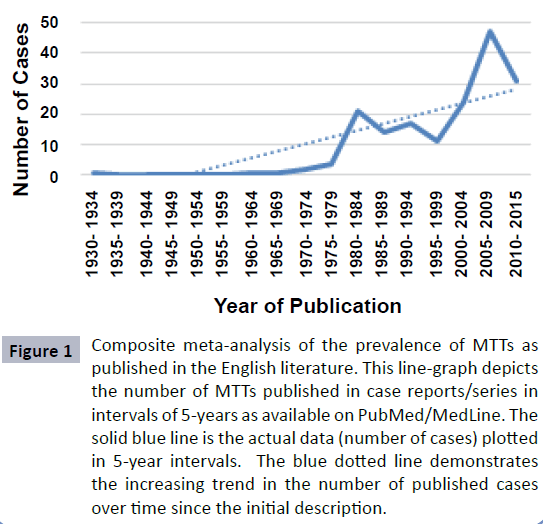
A total of 136 articles were identified, from the first description of triton tumors in 1932 to the present. As demonstrated in Figure 1, rates of MTT reported in the literature have been steadily increasing since the 1960s; this may be due to greater clinical awareness of the tumor, improved immunohistochemical tests, or increased accessibility to worldwide publications.
Figure 1: Composite meta-analysis of the prevalence of MTTs as published in the English literature. This line-graph depicts the number of MTTs published in case reports/series in intervals of 5-years as available on PubMed/MedLine. The solid blue line is the actual data (number of cases) plotted in 5-year intervals. The blue dotted line demonstrates the increasing trend in the number of published cases over time since the initial description.
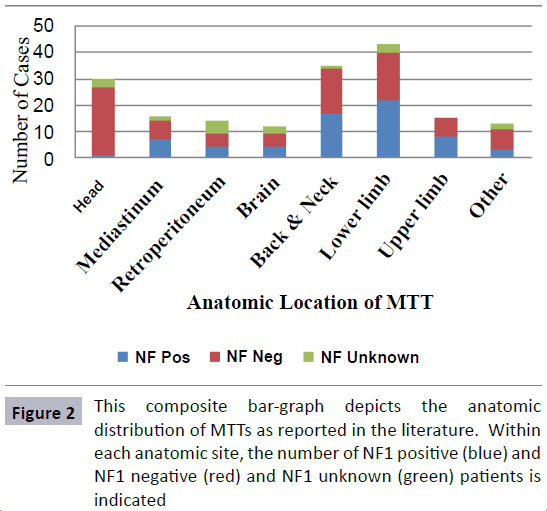
The majority of reported cases were in the lower limb (24%), followed by the back/neck (19.6%), head (16.9%) and mediastinum (8.9%). While in most locations MTT is associated with NF in ~50% of the cases, in the head, only a single case had a positive NF status (Figure 2) which is ascribed as post-radiation neurofibrosarcoma [5]. There is no satisfactory explanation available in the literature for this observed difference.
In all anatomical locations, an almost equal split was observed between NF positive (41.5%) and negative (58.5%) patients; however, NF-positive patients were overall younger (mean 27.69 years vs. 40.5 years) and had a worse prognosis: (22.4% reported overall survival in NF positive vs. 50.6% in NF negative). This data is, however, incomplete as many case reports/series did not report clinical outcomes.
Among the 30 MTTs of the head, 17 involved the nasal/maxillary sinuses and all were NF negative. Patients’ ages ranged from 28- 81 years (average 54.7) and, most interestingly, the mortality rate was very low. Only two patients had died at the time of manuscript publication, one from an unrelated malignancy with no evidence of MTT recurrence, and the other a patient who had non-resectable tumor at initial presentation. All other patients were treated surgically, with eight receiving adjuvant radiotherapy and two adjuvant chemotherapy as summarized in Table 1 [6-16].
| Reference | Age/Sex | Location | Clinical Presentation | NF-1 Status | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Xue[7] | 47/F | Rtparanasal sinus | Facial swelling, nasal obstruction | Unknown | Lateral rhinotomy, resection, radiotherapy | Alive-12 months |
| Shajrawi[8] | 75/M | Lt nasal cavity and sinuses | Recurrent epistaxis | Unknown | Surgical debulking, radiotherapy | AWD-3.5 years |
| Bhatt [9] | 66/F | Lt nasal cavity | Left nasal obstruction, epistaxis | Negative | Extended external ethmoidectomy | AWD-27 months |
| Heffner [10] | 58/M | Lt nasal cavity | Nasal obstruction and mass | Negative | Surgery, radiotherapy | Alive 4 years |
| 56/F | Lt nasal cavity | Nasal obstruction and mass | Negative | Local excision | AWD-22 years | |
| 59/M | Rt nasal cavity | Epistaxis and nasal mass | Negative | Transpalatal excision, radiotherapy | Alive 8.3 years | |
| 43/M | Rt nasal cavity | Rt nasal mass and obstruction | Negative | Unknown | Alive- 22 years | |
| Can [11] | 30/F | Rt maxillary sinus | Progressively enlarging mass | Negative | Radical resection, chemoradiation | Alive-2 years |
| Nicolai [12] | 81/F | Rtethmoid sinus | Nasal obstruction, polypectomy x3 | Negative | Endoscopic excision | Alive-36 months |
| Kim [13] | 38/F | Rt nasal cavity and sinuses | Nasal obstruction, rhinorrhea, headache | Negative | Medial maxillectomy, radiotherapy | Alive -5 years |
| Terzic[6] | 35/F | Nasal cavity | Nasal obstruction, hyposmia, pain | Negative | Subcranial radical resection | Alive -30 months |
| 77/M | Nasal cavity | Nasal obstruction, hyposmia, epistaxis, pain | Negative | Endoscopic resection, radiation (54Gy) | Alive -7.5 months | |
| 73/F | Rt maxillary sinus | Unknown | Negative | Endoscopic resection | Died (melanoma)- 5.5 years | |
| 76/M | Lt nasal cavity | Hyposmia, exophthalmos, blindness | Negative | Refused treatment | Died-1.5 months | |
| Lau [14] | 42/M | Lt nasal cavity | Nasal obstruction | Negative | Left medial maxillectomy, septectomy, craniofacial resection | Alive-lost to follow-up |
| Zakzouk[15] | 49/F | Rt nasal cavity | Epistaxis, nasal obstruction, anosmia | Unknown | Surgical excision, chemoradiotherapy | Alive-5 years |
| Yasuda [16] | 15/F | Rt maxillary sinus | Swelling lateral right nasalala | Negative | Tumor extirpation, radiotherapy | Alive-30 months |
| Locatelli[17] | 26/F | Lt nasal cavity | Nasal obstruction | Negative | Radical resection (ethmoidectomies, middle meatalantrostomies, middle turbinectomies) | Alive-6 years |
Table 1: Triton tumors of the sinonasal tract as reported in the published English literature. AWD: Alive with disease.
Discussion
Historical perspectives
The recognition of triton tumors is based on Locatelli’s study [17] where an implanted sciatic nerve into the back of a Triton salamander developed an accessory limb containing both neural and muscular tissue. Masson was the first to describe what is now referred to as a ‘triton tumor’ in a 23 year-old male with neurofibromatosis [18].
It was, however, not until 1973 that the lesion was first called a “triton tumor” in the published literature so labeled in reference to Locatelli’s salamanders. In this publication, Woodruff described three diagnostic criteria [19]:
• Arises along the course of a peripheral nerve or in a patient with von Recklinhausen’s disease or in a location typical for peripheral nerve tumors (e.g., skin and subcutaneous tissue) or represents a metastasis from such a tumor
• In large part shows growth characteristics of Schwann cells and
• Contains bonafide rhabdomyoblasts
Daimaru expanded Woodruff’s definition of “malignant triton tumor” to include patients not diagnosed with neurofibromatosis, thus including two additional types of tumors [20]:
• Tumors in patients without von Recklinghausen’s disease that are microscopically compatible with malignant schwannoma and contain focal rhabdomyoblastic differentiation
• Tumors consisting predominately of rhabdomyoblastic differentiation with focal Schwann cell elements occurring within a nerve or in patients with von Recklinghausen’s disease
Currently, the WHO classification of tumors of soft tissue and bone recognizes benign and malignant triton tumors; the benign triton tumor, in contrast to the malignant counterpart, is composed of proliferation of nerve fibers admixed with mature skeletal muscle elements [21].
Recently, a new entity, the biphenotypic sinonasal sarcoma (BSNS), has been described as a sarcoma characterized by spindle cell proliferation with both neural and myogenic phenotypes associated with a novel PAX3-NCOA1 fusion. Reporting authors suggest that lesions previously described as “low-grade MPNSTs” such as MTTs of the sinonasal tract may represent BSNS with rhabdomyoblastic differentiation. However, consistent lack of SOX10 immunoexpression in BSNS favors an independent class of tumors, as SOX10 is an essential transcription factor for the development of neural-crest derived cells such as Schwann cells. The increased overexpression of S100 in MTTs clearly favors a Schwannian origin. It is, however, plausible that BSNS may represent one end of the spectrum in the oncogenesis of sinonasal malignant triton tumors. Additional research to evaluate this pathway is warranted [22].
Etiology
The precise etiology of these tumors is not fully understood. Theories of etiopathogenesis include: a) Schwann cells or neural crest cells induce muscular differentiation of other endoneurial cells, b) The “metaplastic theory”-malignant Schwann cells (neural crest origin) retain their capacity for mesenchymal differentiation and transform directly into striated muscle cells [2,12].
MPNST is the most common malignancy associated with Neurofibromatosis-1 (NF1) with a lifetime risk of 5-10%; significantly higher than the general population risk of 0.001%. Tumors in NF1 patients occur in axial locations in young men and are associated with earlier recurrences and metastases [23].
The genetic basis of sporadic MTTs is not yet elucidated. Over half of all MTTs are reported to have translocations/deletions of chromosome 1, with cytogenetic abnormalities in chromosomes 12 and 17 [4]. A breakpoint in 11p15 may be responsible for rhabdomyoblastic differentiation [1,2]; however, no specific cytogenetic factor reliably differentiates MTT from MPNST or explains the difference in natural history or clinical behavior [24]. Additional breakpoints identified in MPNSTs include 1p, 7p22, 11p1323, 20q13, and 22q1113; though, the relevance of these molecular changes to MTTs remains unknown [2]. Overexpression of c-myc has been implicated in relation to the aggressive nature of MTTs [1,2]. Recently a fusion gene process has been implicated in related lesions of the sinonasal tract (BSNS) as discussed above.
Clinical
MTTs can arise anywhere in the body; most common in the trunk (32%), extremities (24%), and head/neck (20%) [25]. MTTs most often arise from large nerves such as the sciatic nerve, spinal root, lumbosacral plexus, brachial plexus, and cranial nerves [1,21].
Unlike other anatomic locations where MTTs appear to develop equally in NF-positive and NF-negative patients, sinonasal tumors are almost exclusively limited to NF-negative patients. As suggested in the literature, head/neck MTTs are low-grade lesions with a less aggressive course [25]. Among MTTs of the head, the nasal/maxillary sinuses are the most commonly affected and seem to confer improved prognosis if completely resected with negative surgical margins.
Pathology
Sinonasal MTTs show no unique histopathological features to differentiate from other MTTs. On gross exam these are firm tumors that may be either pseudoencapsulated or with poorly defined margins with focal regions of necrosis and/or hemorrhage [2]. Morphologically these tumors are characterized by skeletal muscle differentiation including the presence of rhabdomyoblasts and a variety of Schwannian features that include: a) alternating regions of hypo and hypercellularity, b) thin wavy comma-shaped nuclei in the hypocellular regions, c) the presence of nuclear palisading, d) the presence of nerve whorls or tactoid bodies resembling Wagner-Meissner corpuscles, and e) thick-walled vasculature. Definitive diagnosis of MTT requires confirmation of skeletal muscle differentiation with immunohistochemistry (myoglobin, desmin, muscle actin, or myo-D1 positivity) [1,2,21]. The proportion of rhabdomyoblastic cells is variable. Positive staining for S-100 in 50-90% percent of MTTs supports the presence of neurogenic elements of Schwannian origin [1]. A small subset of these lesions may have additional epithelial/mesenchymal elements, thus having “pluri-directional differentiation” [12]. Recently, due to the identification of a novel PAX3-NCOA1 fusion in BSNS, demonstration of PAX3 genetic abnormalities by FISH is proposed as an additional diagnostic tool in its identification [22].
Benign triton tumors, also known as neuromuscular hamartoma, though rare, do exist. They are most often asymptomatic and, like their malignant counterparts, are more aggressive when centrally located compared with non-aggressive peripheral lesions [26]. They are histologically characterized by the presence of well differentiated mature striated muscle admixed with nerve fibers [21].
Treatment
While there are no evidence-based guidelines for the treatment of sinonasal MTTs, consensus based guidelines agree that these nerve sheath tumors are best treated by wide surgical resection with negative free margins and post-operative high-dose radiotherapy for positive margins/residual tumors. The efficacy of neo-adjuvant and adjuvant radiochemotherapy in MTT remains inconclusive [27]; however, as for any sarcoma, resection of the tumor with wide margins followed by radiotherapy is recommended as the standard of care. Radical excision is the only feasible option available for recurrences as the tumor is predominately chemo-refractory. Reconstructive surgical procedures are tailored on an individual case-by-case basis. Based on a small sample sized study, McConnell et al. recommend the use of radiotherapy routinely for all MTT patients irrespective of margin status [24]. Adjuvant chemoradiotherapy may increase survival for completely resected tumors. Proposed targets for the management of MPNSTs including the mTOR inhibitor rapamycin and/or AKT inhibitors, which may be future targeted therapies for MTT [23].
Prognosis
Traditionally, MTTs are reported to have an aggressive biological behavior, with a 5-year survival rate of only 5-15% [1,21], with local recurrence in ~43% and metastases in ~48% [13]. The most common cause of death is metastases to the lung and brain [28]. It is poorly understood why skeletal muscle differentiation increases the aggressiveness of these tumors. These tumors are fast growing and are prone to local recurrence/hematologic metastases [27]. Traditional adverse prognostic factors include truncal location, tumor size >5 cm, local recurrence, NF-1 positivity, high-grade, and completeness of resection with negative surgical margins [1,21].
In contrast to this conventional behavior, MTTs of the sinonasal tract seem to behave as lower-grade neoplasms with increased survival rates. As seen in Table 1 all cases were alive at reported long-term follow-up (up to 22 years).
Conclusion
Historically accepted “truths” about malignant triton tumors may not pertain to these neoplasms in the nasosinuses. It was historically believed these tumors are more likely to occur in patients with NF1 and portend a poor prognosis secondary to its highly aggressive natural history. Our review suggests that sinonasal MTTs arise sporadically in non-NF1 patients and are associated with improved prognosis and higher survival rates. These observations herald significant clinical questions including the role of routine postoperative adjuvant therapy in sinonasal MTTs. Currently, the distinction of sinonasal MTT vs. BSNS remains an academic nomenclature debate, as there are no overt differences in therapeutic decisions and clinical outcomes in this anatomic region. The necessity for routine long-term surveillance including regular yearly PET-CT and/or biopsies need to be reevaluated. As c-myc mutations may be implicated in the aggressiveness of MTTs, differential expression of this molecular marker in the less-aggressive sinonasal MTTs is recommended for future studies/clinical trials to determine its role as an independent prognostic marker. Finally, due to the rarity of these lesions, a central registry is advised for improved analysis of therapeutic options.
References
- Shetty PK, Baliga SV, Balaiah K(2013) Malignant triton tumor: a rare case.Indian J Surg 75: S362-S365.
- Stasik CJ, Tawfik C(2006) Malignant peripheral nerve sheath tumor with rhabdomyosarcomatous differentiation (malignant triton tumor).Arch Pathol Lab Med 130: 1878-1881.
- Prieto R, Pascual JM, Garcia-Cabezas MA, Lopez-Barea F, Barrios L, et al.(2012) Low-grade malignant triton tumor in the lumbar spine: a rare variant of malignant peripheral peripheral nerve sheath tumor with rhabdomyoblastic differentiation.Neuropathology 32: 180-189.
- Ellison DA, Corredor-Buchmann J, Parham DM, Jackson RJ(2005) Malignant triton tumor presenting as a rectal mass in an 11-month-old.Pediatric and Developmental Pathology 8: 235-239.
- Ducatman BS, Scheithauer BW (1983) Post-irradiation neurofibrosarcoma. Cancer 51: 1028-1033.
- Terzic A, Bode B, Gratz KW, Stoeckli SJ (2009) Prognostic factors for the malignant triton tumor of the head and neck.Head Neck 31: 679-688.
- Xue T, Wei L, Qiao L, Zha DJ, Chen XD (2009) Malignant triton tumour of right paranasal sinuses: case report.The Journal of Laryngology and Otology 123: e16.
- Shajrawi I, Podoshin L, Fradis M, Boss JH(1989) Malignant triton tumor of the nose and paranasal sinuses: a case study.Human Pathology 20: 811-814.
- Bhatt S, Graeme-Cook F, Joseph MP, Pilch BZ (1991) Malignant triton tumor of the head and neck.Otolaryngol Head Neck Surg 105: 738-742.
- Heffner DK, Gnepp DR (1992) Sinonasal fibrosarcomas, malignant schwannomas, and “triton” tumors.Cancer70: 1089-1101.
- Can Z, Saray A, Yilmaz S, Erçöçen AR, Emiroglu M (1999) Malignant triton tumor of the maxilla: a patient report.Ann Plast Surg 42: 96-99.
- Nicolai P, Tomenzoli D, Berlucchi M, Facchetti F, McRassi L, et al. (2000) Malignant triton tumor of the ethmoid sinus and nasal cavity.Ann Otol Rhinol Laryngol109: 880-886.
- Kim ST, Kim CW, Han GC, Park C, Jang H(2001) Malignant triton tumor of the nasal cavity. Head Neck 23: 1075-1078.
- Lau OD, Nabili V, Lai C(2010) Pathology Quiz Case 1.Diagnosis: Sinonasal malignant Triton tumor (MTT) with intracranial extension.Arch Otolaryngol Head Neck Surg 136: 929-930.
- Zakzouk A, Hammad F, Langlois O, Aziz M, Marie JP, et al. (2014) Malignant triton tumour of the sinonasal tract: case report and literature review.Int J Surg Case Reports5: 608-612.
- Yasuda M, Muto Y, Kuremoto T, Murakami K, Onisihi T, et al. (2016) A case of recurrent malignant triton tumor successfully treated with radiotherapy.Auris Nasus LarynxEpub ahead of print.
- Locatelli P (1925) Formation de membres surnuméraires.CR Assoc Des Anatomistes 20e Reunion.Turin, pp: 279-282.
- Masson P (1932) Recklinghausen’s neurofibromatosis, sensory neuromas and motor neuromas.The International Press, New York, USA.
- Woodruff JM, Chernik NL, Smith MC, Millett WB, Foote FW(1973) Peripheral nerve tumors with rhabdomyosarcomatous differentiation (malignant “triton” tumors).Cancer32: 426-439.
- Daimaru Y, Hashimoto H, Enjoji M (1984) Malignant “triton” tumors: a clinicopathologic and immunohistochemical study of nine cases.Hum Pathol15: 768-778.
- Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F (2013)Nerve Sheath Tumors. WHO Classfication of Tumours of Soft Tissue and Bone.Geneva, WHO Press,p: 5.
- Huang SC, Ghossein RA, Bishop JA, Zhang L, Chen TC, et al.(2016) Novel PAX3-NCOA1 fusions in biphenotypic sinonasal sarcoma with focal rhabdomyoblastic differentiation.Am J Surg Pathol40: 51-59.
- Thway K, Fisher C (2014) Malignant peripheral nerve sheath tumor: pathology and genetics.Annals of Diagnostic Pathology18: 109-116.
- McConnell YJ, Giacomantonio CA (2012) Malignant triton tumors-complete surgical resection and adjuvant radiotherapy associated with improved survival.J Surg Oncol106: 51-56.
- Victoria L, McCulloch TM, Callaghan EJ, Bauman NM (1999) Malignant triton tumor of the head and neck: a case report and review of the literature.Head Neck21: 663-670.
- Bae DH, Kim CH, Cheong JH, Kim JM(2014) Adulthood benign triton tumor developed in the orbit.J Korean Neurosurg Soc 56: 146-148.
- Mae K, Kato Y, Usui K, Abe N, Tsuboi R(2013) A case of malignant peripheral nerve sheath tumor with rhabdomyoblastic differentiation: malignant triton tumor.Case Reports in Dermatology5: 373-378.
- Rekhi B, Jambhekar NA, Puri A, Agrawal M, Chinoy RF (2008) Clinicomorphologic features of a series of 10 cases of malignant triton tumors diagnosed over 10 years at a tertiary cancer hospital in Mumbai, India.Annals of Diagnostic Pathology12: 90-97.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences